Technology
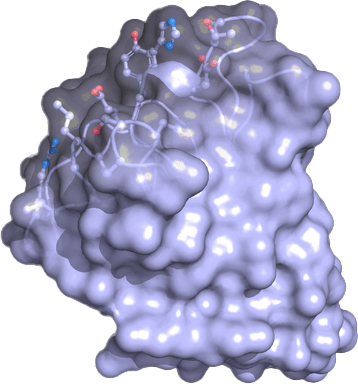
A unique antibody platform
Our platform technology uses camelid antibody fragments (VHH) as building blocks to create novel antibodies for the prevention and treatment of infectious diseases. The platform enables a rapid and robust response to existing and future public health threats:

Multi-specific constructs target multiple epitopes at the same time, making the constructs robust against fast-moving mutations.

VHH can bind “hidden” epitopes that are typically well conserved, resulting in broad specificity.
The resulting therapeutics are stable and cost-effective and easy to produce, facilitating global access.
What are VHH?

Conventional antibodies are comprised of 2 heavy chains and 2 light chains of amino acids.

Camelids also produce a different kind of antibodies, consisting of only 2 heavy chains.
Their specificity is determined by a single variable domain (VHH), which retains its antigen-binding potential as a separate fragment.
MODULAR MULTI-SPECIFIC MEDICINES
Thanks to their small size, VHH can be used as building blocks that are linked using a human Fc region – corresponding to the “tail” of a conventional antibody – as a backbone.
These constructs can combine multiple VHH (with the same and/or a different specificity) in different ways.
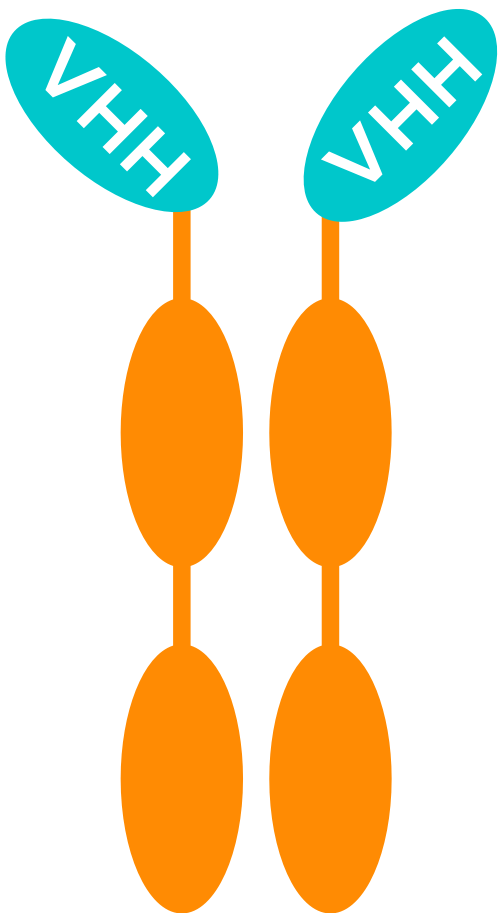

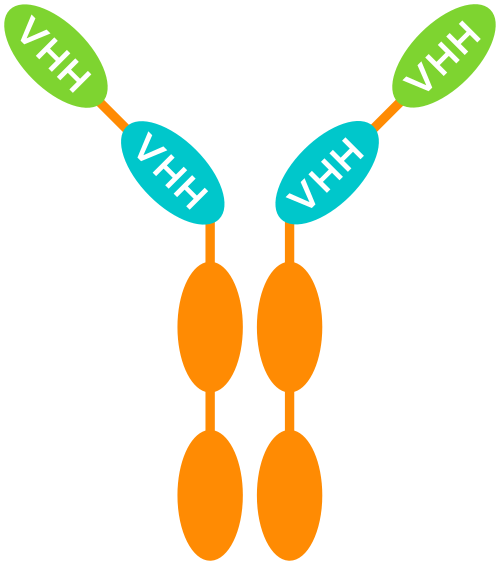
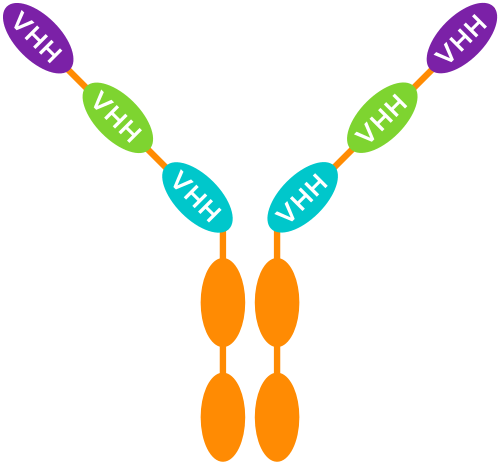
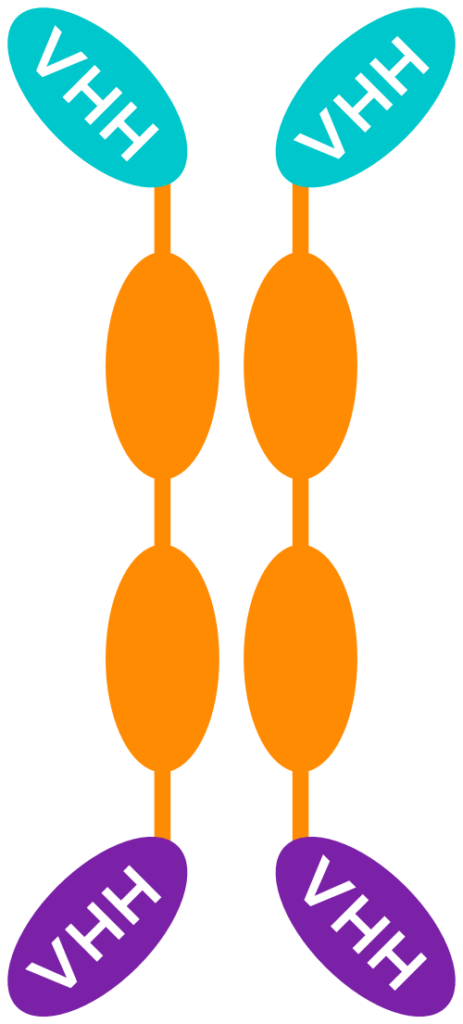
UNIQUE BENEFITS

SPECIFICITY
Thanks to their small size and unique structural properties, VHH can bind epitopes that are inaccessible to conventional antibodies.
Such “hidden epitopes” are often well-conserved between variants and subtypes, giving the VHHs that bind them a broad specificity.

By targeting multiple epitopes simultaneously, a pathogen must evolve each of them to acquire resistance.
Combining specificities in a single molecule is superior to using antibody cocktails, as the manufacturing cost is lower and obtaining regulatory approval is less complex.

DOSING
VHH are quickly cleared by the kidneys. Linking them to an Fc region increases their serum half-life, meaning lower doses or less frequent administration are needed.

In addition to the ability of the VHH to prevent infection, the Fc region induces an immune response against the pathogen. The strength of this response can be fine-tuned by adapting the sequence of the Fc region.

Their favorable solubility, stability, and biodistribution profiles enable faster and better tissue penetration than conventional antibodies.
They can be synthesized using microbial systems such as Pichia, generating high yields of homogeneous products.

POTENCY
The presence of two identical VHH in bivalent constructs improves binding.
