Bio International Convention – June 5-8, 2023 – Boston, USA

Our CEO and COO are attending this partnering event in person to explore opportunities to collaborate in delivering new medicines to prevent and treat infectious diseases in patients.
Antibody Engineering & Therapeutics Europe 2023 – June 6-8, 2023 – Amsterdam, The Netherlands

Our Head of Preclinical Development is attending this event in person to meet the antibody community.
ExeVir Bio Announces New Publication from VIB Scientists Showing Potent and Broad Neutralizing Activity and Infection Protection against SARS-COV-2 and related coronaviruses
ExeVir, which is developing single domain antibody therapies providing broad protection against viral infections, announces publication in BioRxiv of a paper by VIB scientists showing, in in vivo mouse and hamster models, highly potent viral neutralizing activity, protection against infection by SARS-COV-2 and minimized development of alveolar [lung] damage, for XVR011, its unique Llama-derived VHH72-Fc antibody for potential treatment and prevention of Covid-19.
ExeVir Bio Appoints Michel Kazatchkine and Stef Heylen to the Board of Directors

ExeVir, which is developing nanobody therapies providing broad protection against viral infections, including a lead candidate for coronaviruses, today announced that Michel Kazatchkine and Stef Heylen have been appointed to the Board. Both strengthen ExeVir’s Board by adding pertinent R&D, in depth infectious disease and significant global health experience as ExeVir moves from preclinical to clinical with its lead candidate XVR011.
ExeVir Bio to Accelerate Development of New Treatment Conferring Broad Protection Against Covid-19
ExeVir Bio announces today a first closing of a EUR23 million Series A financing led by Fund+, with the participation of VIB, UCB Ventures, the Belgian Federal Government via SFPI-FPIM, V-Bio Ventures and several Belgian family offices. ExeVir Bio has been established by Belgian partners combining world class science, antibody engineering, manufacturing, blue-chip venture capital investment and Flemish Government financing, which have joined forces in a unique collaboration to boost the development of new therapies to combat Covid-19
Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies
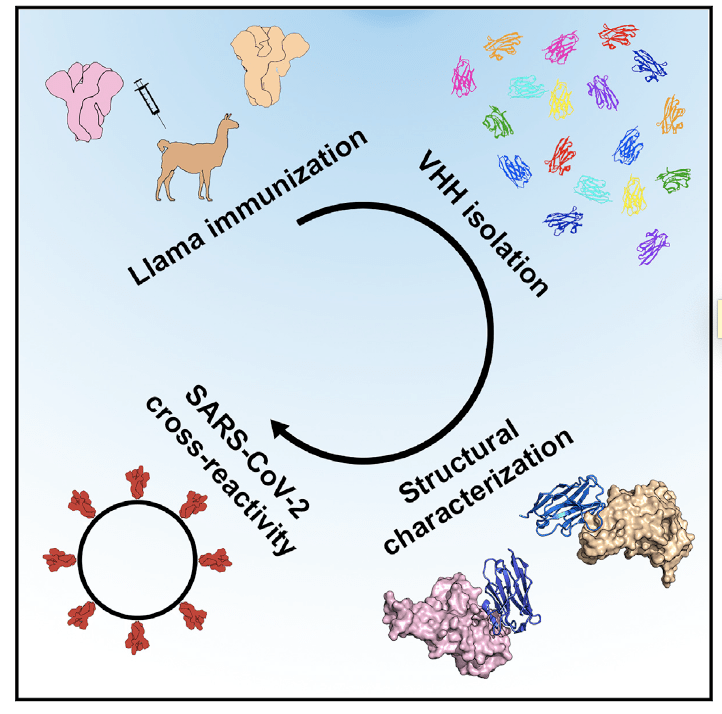
Using llamas immunized with prefusionstabilized betacoronavirus spike proteins, Wrapp et al. identify neutralizing cross-reactive single-domain camelid antibodies, which may serve not only as useful reagents for researchers studying the viruses causing MERS, SARS, and COVID-19, but also potential therapeutic candidates. Crystal structures further reveal how these antibodies bind spike proteins to prevent virus entry into cells.
